When conducting experiments with 96-well plates, proper labeling is crucial to avoid costly mistakes. Without clear and accurate labels, it’s easy to mix up samples, lose track of essential data, and struggle with reproducibility. Whether you’re conducting PCR tests, cell culture assays, or high-throughput screenings, ensuring your 96-well plates are correctly labeled can save you time and help you produce reliable results. This guide will take you through the best practices for marking, orientation, barcodes, and sample tracking, ensuring that your experiments run smoothly.
What Information Should a 96-Well Plate Label Include?
Properly labeling a 96-well plate involves more than just marking the plate with a pen. Each label should contain essential information to help you identify the samples, track experimental conditions, and manage the plate during the experiment. Clear labeling not only minimizes the risk of mix-ups but also ensures that your data is traceable and reproducible.
Essential Metadata
When labeling your 96-well plate, make sure to include the following key details:
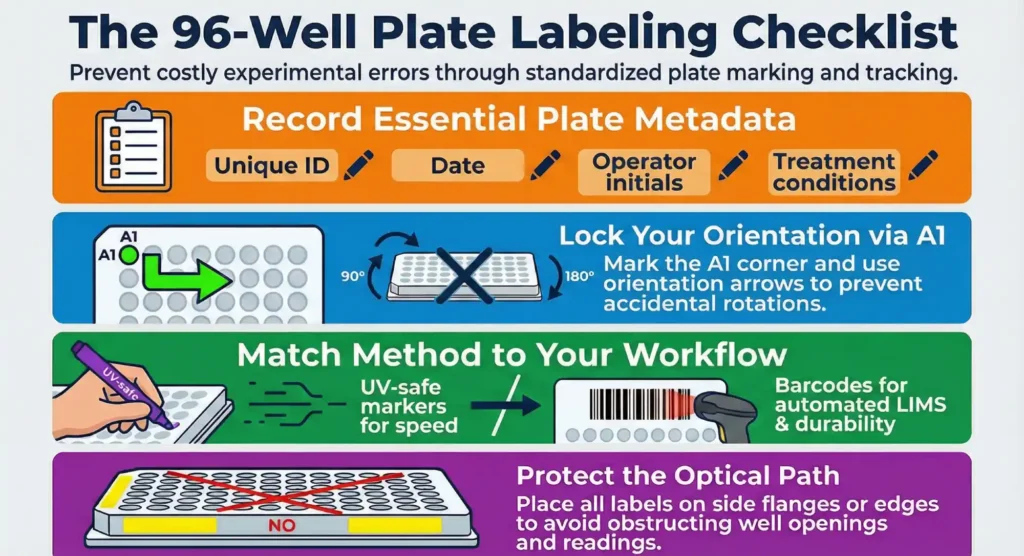
- Plate ID: A unique identifier for each 96-well plate. This helps keep track of individual plates when handling multiple samples.
- Date: Label the date of the experiment to track when it was performed. This is especially useful for long-term studies.
- Operator Information: Initials or identification of the person experimenting. This can be helpful if you need to identify potential errors or consult the person who handled the plate.
- Treatment/Condition: Clearly mark what treatment or experimental condition the plate represents (e.g., drug concentration, cell type, or control vs test group).
- Plate Number: In studies with multiple plates, numbering them will help you quickly locate and reference the right one.
- Replicate Number: If your experiment involves multiple replicates, ensure the replicate number is labeled to distinguish between them.
Well-Level vs Plate-Level Labeling
- Plate-Level Labeling: Typically used for high-level information like plate ID, experiment name, date, and operator initials. This is vital for general identification.
- Well-Level Labeling: Used when you need to specify more details for individual wells, such as sample ID or treatment condition. It’s crucial in cases where the contents of individual wells vary, such as in dose-response experiments.
Including this information ensures full traceability, allowing you to follow the experiment from the beginning to the final data analysis phase.
Plate Orientation & Marking Best Practices
Correctly marking the orientation of your 96-well plate is essential to prevent confusion during experiments. The orientation determines the placement of samples in each well, so if the plate is accidentally rotated, it can result in misinterpretation of the data.
How to Mark Plate Orientation
Orientation should be marked clearly and consistently on all 96-well plates. Here are some best practices for doing so:
- A1 Corner: Always mark the corner of the plate where A1 is located. This is the starting point for your plate, and identifying this corner ensures the plate is always oriented correctly.
- Row and Column Labels: Use large, clear labels along the top and left sides of the plate. Rows are labeled A–H, and columns are labeled 1–12, which creates a grid system for easy identification of well positions (e.g., A1, B12, H8).
- Orientation Arrows: Adding an arrow or line next to the A1 corner helps indicate the top of the plate. This makes it easier to identify the correct orientation even if you have to rotate the plate during handling.
- UV-Safe Markers: If you’re using a permanent marker to label your plate, ensure it is UV-safe. Some markers can fade or smudge under UV light or over time, which can cause confusion in long-term experiments.
Common Orientation Mistakes
- Rotating the Plate: One of the most common mistakes is accidentally rotating the plate 90 or 180 degrees. Always double-check the orientation before starting your experiment and before placing the plate in the reader.
- Flipping the Plate: If the plate is upside down or the top is mistaken for the bottom, the rows and columns can become confused. This can be particularly problematic in assays where precise sample positioning is crucial.
To avoid these errors, consistently label the plate’s corners and edges to ensure that the orientation is easy to identify, no matter where the plate is placed.
Labeling Methods: Pros and Cons
There are several ways to label a 96-well plate, and the method you choose will depend on factors like the scale of your experiment, the duration of storage, and the tools available to you. Here’s a breakdown of some of the most common labeling methods, along with their benefits and drawbacks.
Manual Permanent Markers / Lab-Grade Markers
Using a permanent marker is the simplest method for labeling 96-well plates. It’s quick, cost-effective, and easy to do without specialized equipment. However, there are a few considerations:
- Pros: Inexpensive and accessible; quick for small-scale experiments; works well for marking plate ID and orientation.
- Cons: Permanent markers can fade over time, especially under UV light. They can also smudge if touched before thoroughly drying, and they can be challenging to read if used in excess.
Adhesive Labels / Stickers
Adhesive labels are another standard method for labeling 96-well plates. These labels can be printed with all necessary metadata, such as sample IDs, experiment conditions, and even barcodes. Adhesive labels are more durable than manual markers but come with their own challenges:
- Pros: Clear and professional appearance; can hold a lot of data; can be printed directly from your computer for easy customization; labels can be scanned for digital records.
- Cons: If the labels aren’t applied correctly, they can peel off or obstruct well openings, interfering with optical readings. They can also be prone to fading or tearing under harsh lab conditions (e.g., extreme temperatures, exposure to solvents).
Direct Printing / Barcode Printing
Printing labels directly onto the plate or onto a side flange using specialized printers is a more advanced method, often used in high-throughput or clinical settings. This approach can also include barcodes for sample tracking.
- Pros: Highly reliable and durable; reduces human error; suitable for automation; barcodes allow easy scanning and integration with Laboratory Information Management Systems (LIMS).
- Cons: Requires investment in printing technology and setup; limited flexibility to change labels once printed.
Each labeling method serves a different need, so selecting the right one depends on the complexity of your experiment and your lab’s requirements.
Summary of Labeling Methods
| Labeling Method | Pros | Cons |
| Permanent Markers | Quick, cost-effective, no special equipment | Fading, smudging, and difficult to read |
| Adhesive Labels | Professional appearance, can hold lots of data | Risk of peeling, obstructs well openings |
| Direct Printing/Barcodes | Durable, suitable for automation, and accurate | Requires printing equipment, limited flexibility |
Barcode Labeling & Sample Tracking: For Labs and High‑Throughput Workflows
In modern laboratories, barcode labeling and sample tracking are essential for ensuring data integrity, reducing errors, and increasing workflow efficiency. Barcode labels provide a reliable way to track samples from the moment they are prepared until the results are recorded, making them an invaluable tool for labs, especially in high-throughput or clinical settings. This section will explore how barcode labeling works, the types of barcodes you can use, and how to integrate barcode data into your laboratory’s sample tracking system.
Why Barcode Labeling Is Increasingly Used in Labs
Barcode labels are gaining popularity in laboratories due to their ability to streamline processes, reduce manual errors, and improve traceability. Here are some key reasons why barcode labeling is crucial:
- Automation and Efficiency: Barcode scanning automates the identification and recording of sample information, reducing the risk of human error associated with manual labeling or data entry. This is particularly beneficial in high-throughput labs where hundreds or even thousands of samples are processed.
- Increased Traceability: Barcodes allow you to easily track the movement of samples through various stages of an experiment. This traceability is essential for maintaining detailed records of experimental conditions, sample handling, and results.
- Data Integrity: By using barcodes, labs can ensure that the data associated with each sample is consistently accurate and tied to the correct well or test tube. This minimizes the risk of sample mix-ups or data entry errors.
Recommended Barcode Types
When choosing a barcode system for 96-well plates, it’s essential to consider the plate size, the amount of data required, and the scanning system used. Here are two of the most commonly used barcode types for labeling 96-well plates:
- 1D Barcodes (e.g., Code 128): These are linear barcodes that store a limited amount of data. They are simple to scan and widely used in laboratories. A typical 1D barcode can store information like a plate ID or a sample ID.
- 2D Barcodes (e.g., Data Matrix or QR Code): These barcodes can store more data, such as multiple sample identifiers or experiment conditions. 2D barcodes are highly compact, allowing them to fit on small surfaces like the side flange of a 96-well plate, making them ideal for high-density data storage.
Where to Place Barcodes on 96-Well Plates
The placement of barcodes on your 96-well plate is crucial for both readability and convenience. Here are some best practices:
- Top Edge: The most common placement for barcodes is along the top edge of the plate. This makes it easy to scan the barcode without obstructing the view of the wells, which is especially important when running assays that require optical measurements.
- Side Flange: For plates with multiple labels or for systems that use 2D barcodes, placing the barcode on the side flange allows for easy scanning while keeping the well openings unobstructed.
Avoid Obstructing Wells: Ensure barcodes are positioned so they do not cover or block any wells. This is particularly important if you need to read the contents of the wells, as specific assay techniques require unobstructed access to the plate’s surface.
Barcode Integration with LIMS and Sample Tracking Systems
One of the main benefits of barcode labeling is its integration with Laboratory Information Management Systems (LIMS). LIMS allows laboratories to manage and track data automatically by linking each barcode label to detailed sample information. This integration improves efficiency and data accuracy, as sample information can be scanned, recorded, and updated in real-time. Here’s how barcode integration works:
- Barcode Scanning: Once a 96-well plate is labeled with a barcode, it can be scanned by a barcode reader at various stages of the experiment. This automatically records the plate’s ID, sample data, and other relevant metadata into the LIMS.
- Tracking: As the sample moves through different stages (e.g., preparation, analysis, incubation), its status is updated in the system. This ensures that you can track the exact location and condition of each sample at any point in time.
- Data Synchronization: When the plate is ready for analysis (e.g., a plate reader), the LIMS can be synchronized with the analytical equipment to ensure that the right data is being collected and associated with the correct sample.
- Audit Trail: Barcode labeling creates an automatic audit trail. Each scan is recorded, providing a comprehensive history of the sample, which is essential for ensuring compliance with regulatory standards and for maintaining data integrity.
Validating Barcodes and Ensuring Readability
To ensure your barcodes work correctly, test them regularly for readability, especially under lab conditions. Factors such as environmental lighting, temperature, and plate handling can all affect barcode scanning accuracy. Here are some tips to ensure reliable barcode scans:
Test Readability: Always test barcodes before using them in experiments. Use a barcode scanner to verify that the code is readable under typical lab conditions (e.g., in the incubator, freezer, or on a workbench).
Check for Smudging: Barcodes can be smeared or obscured by liquids, stains, or condensation, so ensure they remain clear and legible throughout the experiment.
Durability: For long-term storage, ensure that your barcode labels are made from durable materials that can withstand freezing, thawing, and exposure to chemicals.
By implementing barcode labeling and integrating it with your lab’s sample tracking system, you can improve workflow accuracy and efficiency while reducing the risk of human error.
Sample Tracking & Documentation Workflow
Once a 96-well plate is labeled with the necessary metadata and barcodes, it’s crucial to establish an organized system to track the plate and its contents throughout the experiment. Proper sample tracking ensures that every well is associated with the correct data, experiment conditions, and results. This section will cover best practices for creating a robust sample-tracking and documentation system.
Building a Plate Log
A plate log is a vital part of your sample tracking system. It acts as a record of all the plates used in an experiment, ensuring that you can trace the history of each plate from start to finish. Here’s what your plate log should include:
- Plate ID: This should match the barcode or label on the 96-well plate to ensure you can cross-reference it easily.
- Date and Time: Documenting when the plate was created or processed helps track experimental timelines and control for potential confounding factors like time-dependent effects.
- Experiment Details: Include a brief description of the experimental conditions or treatments applied to the plate (e.g., drug dose, assay type).
- Sample List: For each well on the plate, document the sample type or identification (e.g., sample ID, replicate number, control or test group).
- Barcode/Label ID: Record the barcode or unique label associated with the plate. This is critical for integrating the physical plate with your digital records.
Record-Keeping Best Practices
To ensure your sample tracking system is reliable, here are some best practices:
- Use Digital Records: Maintain a digital version of the plate log for easy access and management. Spreadsheet programs or specialized LIMS can help keep the data organized and secure.
- Sync with Experimental Data: As data is generated (e.g., from plate readers or other analytical instruments), link it back to the corresponding plate log entry. This ensures that all data is associated with the correct samples.
- Back-Up: Always keep a backup of your sample tracking records in case of data loss or technical failure. This is particularly important in regulated environments where traceability is critical.
Standard Operating Procedures (SOPs)
Standardizing your sample tracking practices is key to ensuring consistency across experiments. Develop an SOP that outlines the steps for labeling, logging, and tracking 96-well plates. Include the following:
- Labeling Protocol: Establish a consistent approach to labeling all plates, including the use of barcodes, metadata, and orientation markings.
- Documentation Requirements: Define what needs to be recorded for each plate and well. This includes sample IDs, experimental conditions, and any special handling instructions.
- Quality Control: Establish procedures to verify the accuracy of labels and records before starting experiments. This helps catch errors before they affect your data.
By implementing a solid documentation and tracking workflow, you ensure that your experiments are organized, reproducible, and free of errors that could compromise your results.
Common Mistakes & How to Avoid Them
Even experienced lab technicians can make mistakes when labeling 96-well plates, leading to costly errors and compromised data integrity. The 96-well plate layout is designed for consistency and efficiency. Identifying these common mistakes and understanding how to avoid them is essential for maintaining reliable and reproducible results. Here are some of the most frequent labeling errors and how to prevent them.
1. Label Smudging or Fading
One of the most common issues with manual labeling is smudging or fading. This often occurs when permanent markers or non-durable adhesive labels are used, especially when the plate is exposed to moisture, heat, or UV light.
How to Avoid:
- Use UV-safe markers that are designed to withstand exposure to light and chemicals.
- For greater durability, consider using printed labels or directly printing onto the plate. This eliminates the risk of smudging and ensures that the label remains readable throughout the experiment.
- Choose water-resistant labels if using stickers, and apply them properly to avoid peeling or damage.
2. Labels Blocking Wells or Interfering with Optical Readings
Some labels may cover the well openings, interfering with optical readings during assays like ELISA or PCR. This can distort data, as the optical path may be blocked or the wells may not be detected correctly by the equipment.
How to Avoid:
- Place labels on the edges of the plate, either on the flange or around the periphery, making sure the wells remain unobstructed.
- If using barcodes, position them on the side flange or top edge of the plate to prevent them from covering any wells. This keeps the plate ready for optical readings without obstructions.
3. Mixing Up Plate Orientation
Mixing up the orientation of a 96-well plate is a frequent mistake. If the plate is rotated or flipped without proper identification, the rows and columns can become mismatched, leading to incorrect sample analysis.
How to Avoid:
- Always mark the A1 corner with a clear identifier (e.g., a permanent marker or orientation arrow) to indicate the correct orientation.
- Place a permanent orientation mark on the plate lid as well. This ensures that both the plate and the lid align correctly when handled or stored.
- Train laboratory personnel to verify orientation before moving the plates between stages of the experiment.
4. Barcode Smudging or Unreadable Barcodes
Barcode labels are handy for sample tracking, but they can become unreadable due to smudging, condensation, or improper placement. This can halt the entire workflow, especially in automated labs relying on barcode scanning for data entry.
How to Avoid:
- Use high-quality, durable barcode labels made for laboratory environments. These labels should be resistant to smudging and tearing.
- Ensure that barcodes are placed on flat surfaces, such as the side flange or the top edge, and are not exposed to excessive handling or solvents.
- Test barcode readability regularly to ensure it remains scannable throughout the experiment. It’s crucial to validate barcodes under real conditions (e.g., in the incubator, freezer, or with reagents applied) to ensure they will scan accurately.
5. Reusing Plates Without Relabeling
In some cases, researchers may reuse 96-well plates without updating the labels. This can lead to sample cross-contamination or misidentification if the old labels or metadata are still visible or haven’t been corrected.
How to Avoid:
- Relabel plates after every experiment, even if the plate is reused. Clear out the old labels and reapply new ones with updated metadata.
- For long-term reuse, consider using a plate washing system or disposable labels that can be easily removed between uses.
FAQs
How do I mark the orientation of a 96-well plate?
To mark the orientation, ensure that the A1 corner is clearly marked. Use an arrow or permanent marker on the top-left corner of the plate to indicate its orientation.
Can a Sharpie marker be used to label plates?
While a Sharpie can be used, it’s better to use UV-safe, laboratory-grade markers that won’t fade under exposure to light or solvents.
How do I choose between adhesive labels and barcodes?
For small-scale or manual experiments, adhesive labels are fine. For high-throughput experiments, barcodes are preferable, as they can be scanned for automatic data entry and integration with a LIMS.
Can I reuse 96-well plates?
Yes, but relabel the plates each time they are reused to avoid mix-ups. Ensure that old labels are entirely removed, and new metadata is applied.
Final Thoughts & Recommendations
Correct labeling is essential for accurate sample tracking and reproducibility in 96-well plate experiments. By following these best practices for marking orientation, using barcodes, and keeping detailed sample logs, you’ll reduce the risk of errors and improve the overall efficiency of your lab. For larger or automated labs, investing in barcode printing and LIMS integration will streamline your workflow and ensure smooth data management. Proper labeling ensures that your experiments are transparent, reproducible, and traceable, leading to more reliable scientific outcomes.
If you need a downloadable template or further resources on creating labeling systems for 96-well plates, feel free to get in touch.

